-
Why Infusion Filters Are Essential in Modern IV Therapy
Views: 126 Author: Site Editor Publish Time: 2025-06-13 Origin: Site

Intravenous (IV) therapy is fundamental in healthcare, delivering fluids, medications, and nutrients directly into patients' bloodstream. Yet, IV therapy carries risks—particulate contamination, bacterial infection, and air embolism—that can have serious consequences. Infusion filters, small devices integrated into IV lines, serve as the critical last line of defense against these hazards. Selecting the right infusion-filter supplier isn’t merely a procurement task; it's a strategic decision that directly impacts patient safety and clinical efficiency.
Why Supplier Selection Matters
Infusion filters protect patients by trapping tiny particles (rubber fragments, glass shards, drug precipitates), blocking microbial contaminants, and eliminating air bubbles. A single defective filter can lead to phlebitis, sepsis, or air embolism, causing harm, increasing liability, and disrupting operations. Hospitals must therefore view filters not as commodities but as essential medical devices requiring rigorous vetting. Beyond price, procurement teams should evaluate suppliers based on regulatory compliance, material quality, production transparency, and after-sales support. A reliable partner delivers consistent, certified quality while offering the flexibility and responsiveness needed to adapt to evolving clinical demands.
Key Criteria: Certifications, Materials, Traceability
Certifications: Demonstrating Compliance and Safety
ISO 13485 Certification: This international standard ensures a medical-device manufacturer’s quality management system covers design, production, and post-market activities. ISO 13485–certified suppliers follow documented procedures for risk assessment, corrective actions, and process validation, guaranteeing consistent filter performance.
CE Marking (Europe): CE-marked infusion filters comply with EU health, safety, and environmental requirements. Achieving CE approval involves biocompatibility testing (ISO 10993), performance verification (flow-rate and bubble-point tests), and package integrity validation.
FDA 510(k) Clearance (United States): In the U.S., most infusion filters are Class II devices, requiring 510(k) premarket notification to demonstrate substantial equivalence to an existing legally marketed filter. Securing FDA clearance indicates robust regulatory expertise.
Other Regional Approvals: Suppliers aiming for global distribution may also obtain ANVISA (Brazil), PMDA (Japan), or NMPA (China) approvals. Multiple international certifications simplify procurement for distributors serving diverse markets.
Materials: Ensuring Biocompatibility and Durability
Membrane Materials:
Polyethersulfone (PES): Offers high flow rates and minimal protein binding, making it ideal for protein-containing infusions (e.g., albumin, antibiotics).
Polypropylene (PP): Provides excellent chemical compatibility with lipid emulsions and parenteral nutrition, though typically with larger pore sizes (e.g., 1.2 microns).
Housing Polymers: Medical-grade polycarbonate (PC) or ABS withstand sterilization (gamma irradiation or ethylene oxide) without leaching toxins, resist cracking under negative pressures, and maintain transparency where needed.
Gaskets and Seals: Silicone or fluorosilicone components ensure hygienic, leak-free connections without reacting chemically with IV fluids.
Coatings and Additives: Filters for protein-rich or lipid-rich infusions may feature hydrophilic or hydrophobic coatings (e.g., polyethylene glycol) to reduce clogging and maintain flow rates during extended use.
Traceability: Linking Every Filter to Its Origin
Unique Lot Numbering: Each filter carries a batch/lot number, linking it to specific raw materials, production runs, and sterilization cycles.
Detailed Production Records: Suppliers maintain records—raw-material certificates, in-process test results (flow-rate, bubble-point), sterilization logs (ISO 11137 or ISO 11135), and final inspections—readily available for audits or recalls.
Expiration and Sterility Documentation: Filters display manufacture (MFG) and expiration (EXP) dates. Sterilization validation ensures a Sterility Assurance Level (SAL) of 10^–6.
Recall Procedures: In the event of any deviation (e.g., failed sterility testing), documented recall protocols enable swift identification, notification, and quarantine of affected batches.
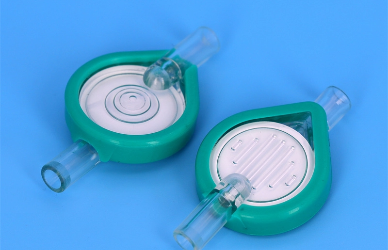
Importance of Consistent Quality and Supply Chain Stability
Rigorous Quality Control
Incoming Inspection: Medical-grade polymers and membranes undergo acceptance testing—verifying membrane thickness, polymer tensile strength, and bioburden levels—before entering production.
In-Process Monitoring: During assembly, bubble-point tests confirm pore integrity, visual inspections detect pinholes or housing defects, and automated systems verify correct gasket placement.
Flow-Rate Testing: Representative filters from each batch undergo simulated clinical infusions (e.g., 20 drops per minute at 200 mmHg) to ensure they meet labeled performance.
Sterilization Validation: In-house or contracted gamma irradiation and ethylene oxide processes follow ISO 11137 or ISO 11135 guidelines, confirming an SAL of 10^–6.
Final Inspection: Post-sterilization checks verify package integrity, accurate labeling (lot codes, expiration dates), and physical appearance. ISO 13485–certified systems bind these steps into a traceable workflow, with any deviation triggering corrective and preventive actions (CAPA).
Supply Chain Resilience
Multiple Sourcing: To avoid single-point failures, top suppliers maintain relationships with multiple membrane and polymer vendors. Should one supplier face delays, alternative sources ensure uninterrupted production.
Buffer Stocks: Maintaining safety inventories of critical components—membranes, resins, packaging—absorbs demand spikes or supplier delays without halting assembly.
Logistics Partnerships: Collaborations with freight forwarders, customs brokers, and local distributors ensure expedited shipping (air freight) and proper handling of specialized filters requiring temperature control.
Regulatory Expertise: Suppliers with local subsidiaries or established distribution partners in key markets (Europe, North America, Southeast Asia) expedite customs clearance and local registration, reducing time to market.
A resilient supply chain prevents filter stockouts, avoiding delays in patient care, surgical procedures, and critical care interventions.
Value-Added Services: OEM/ODM, Technical Support, Logistics
OEM/ODM Customization
Private Labeling and Packaging: Hospitals often require branded packaging—custom labels, hospital logos, or barcodes for electronic inventory systems. OEM services enable tailored carton sizes, localized instruction leaflets, and custom artwork.
Bespoke Filter Designs: Certain clinical scenarios—chemotherapy infusions, high-flow trauma resuscitation, neonatal care—demand unique solutions. ODM collaborations allow the development of custom pore sizes, membrane coatings, or low-dead-space housings to meet these needs.
Integrated IV Kits: To reduce nursing setup time and assembly errors, some suppliers provide prepackaged “IV therapy kits” combining the filter, tubing, drip chamber, and connectors in a single sterile pouch.
Technical Support and Training
Onsite or Virtual Training: Infusion filters require correct priming and installation. Training covers:
Proper air-bubble elimination techniques.
Correct orientation of air-eliminating filters.
Recognizing flow-rate drops indicating occlusion and safe filter replacement.
Compatibility guidelines for various infusates (lipid emulsions, antibiotics, chemotherapeutics).
24/7 Technical Hotline: Immediate guidance on filter performance, troubleshooting occlusions, and clarifying usage instructions ensures uninterrupted patient care.
Resource Libraries: Online repositories of white papers, clinical application notes, and evidence-based best practices help clinical educators develop internal protocols, such as “Filter Selection for Total Parenteral Nutrition vs. Chemotherapy” and “Reducing Catheter-Related Infections.”
Logistics and Inventory Management
Just-In-Time (JIT) Delivery: Collaboration with supplier logistics teams enables frequent, small shipments aligned with consumption rates—minimizing on-hand inventory and freeing up storage space.
Vendor-Managed Inventory (VMI): Suppliers monitor usage data (via EDI or EHR integration) and automatically replenish stock when levels approach predefined thresholds, reducing manual ordering and preventing stockouts.
Global Shipping Expertise: Experienced freight partners handle temperature-controlled shipments for specialized filters (e.g., heparin-coated) and provide comprehensive documentation—certificates of origin, free-sale certificates, and technical files—to facilitate customs clearance.
Contingency Planning: During crises—pandemics, natural disasters, geopolitical tensions—suppliers deploy alternative shipping routes (air cargo vs. sea freight), leverage diversified distribution hubs, and maintain emergency-use inventory in strategic regions to ensure continuous delivery.

ZhenFu Group as a Long-Term Partner: Capabilities & Case Studies
ZhenFu Group, founded in 2005 in Shandong Province, China, has over 15 years of experience in medical filtration and laboratory consumables. Their core strengths include:
Manufacturing Excellence and Regulatory Compliance
ISO 13485:2016 Certification: ZhenFu’s facilities cover membrane fabrication, housing injection molding, assembly lines, and in-house gamma irradiation sterilization. Every stage follows documented protocols—raw-material acceptance, in-process testing (bubble-point, flow-rate), and final inspection—ensuring consistent quality.
CE and FDA 510(k) Approvals: ZhenFu’s infusion filters carry CE marking for EU distribution, while select models have FDA 510(k) clearance for the U.S. market. Dual regulatory expertise simplifies global procurement and minimizes approval lead times.
In-House Sterilization Center: Vertical integration of sterilization reduces lead times and allows real-time dose mapping and biological-indicator tracking to achieve a Sterility Assurance Level (SAL) of 10^–6.
Diverse Product Portfolio and Customization
0.2-Micron Hydrophilic PES Filters: Ideal for aqueous solutions—antibiotics, saline—offering high flow rates (≥ 50 mL/min at 200 mmHg).
1.2-Micron Lipid-Resistant PP Filters: Engineered for parenteral nutrition and lipid emulsions, they resist clogging under high-viscosity conditions.
Air-Eliminating Filters: Featuring dual-chamber designs with hydrophobic vents, these filters automatically trap and purge air bubbles while maintaining a sterile barrier—critical for neonatal and pediatric patients.
OEM/ODM Services: ZhenFu collaborates on custom packaging (private labeling, multilingual instructions) and bespoke filter designs (custom pore sizes, low-dead-space housings) to meet specialized clinical and branding requirements.
All-in-One IV Therapy Kits: Prepackaged kits combine filters, tubing, drip chambers, and connectors in a single sterile pouch—reducing setup time and assembly errors in high-turnover environments.
Case Studies Demonstrating Partnership Value
Southeast Asian Tertiary Hospital: Implemented a Vendor-Managed Inventory (VMI) program with ZhenFu, achieving zero stockouts over 18 months, reducing purchase-order processing time by 40%, and cutting per-unit freight costs by 15%.
German Oncology Center: Partnered with ZhenFu to develop chemotherapy-compatible PES filters with specialized hydrophilic coatings. Over 12 months, the center recorded zero infusion-related complications and reduced filter-change frequency by 20%.
U.S. Pediatric ICU: ZhenFu engineered an ultra-low dead-space filter for neonates (<1 kg), validated with dual-stage low-pressure bubble testing for flows as low as 0.3 mL/hour. A California distribution hub cut shipping times to 5–7 days, and a 24/7 technical hotline ensured immediate support. The unit reported zero air-embolism events over 12 months.
Conclusion
Infusion filters are the final barrier protecting patients from particulates, microbes, and air bubbles during IV therapy. Given the potential for severe complications, hospitals and clinics must treat these filters as essential medical devices rather than disposable commodities. Key considerations for supplier selection include:
Certifications and Regulatory Compliance: ISO 13485, CE marking, FDA 510(k), and other regional approvals accompanied by ISO 10993 biocompatibility and ISO 11137/11135 sterilization validation.
Materials and Manufacturing Transparency: Medical-grade PES or PP membranes, housing polymers (PC, ABS), and rigorous incoming, in-process, and final inspections.
Traceability and Quality Management: Unique lot numbers, detailed production records, and robust CAPA and recall procedures.
Supply Chain Resilience: Multiple sourcing, buffer stocks, strategic logistics partnerships, and regulatory expertise to prevent stockouts.
Value-Added Services: OEM/ODM customization, hands-on training, 24/7 technical support, JIT and VMI inventory models, and global shipping capabilities.
Proven Long-Term Partnership: Real-world case studies demonstrating consistent performance in diverse clinical environments (ICU, oncology, pediatrics).
ZhenFu Group exemplifies these attributes with ISO 13485–certified production, CE and FDA approvals, an in-house sterilization center, a wide range of infusion filters, OEM/ODM customization, and unmatched technical and logistical support. By partnering with ZhenFu, healthcare providers gain more than a supplier—they gain a dedicated collaborator committed to innovation, quality, and responsiveness, ensuring safer IV therapy and better patient outcomes every time.
For further details about ZhenFu Group’s infusion filters and to explore custom solutions, visit www.zhenfugroup.com .
Related Products
CONTACT US
NO.176, Gaoxin 5th Road, High-tech Industrial Park, Rizhao City276800, Shandong Province, China +86-13396234532 +86-13396234532Copyright © 2023 ZhenFu Group All Rights Reserved. Technology By leadong.com | Sitemap | Privacy Policy


















