-
Infusion Filters And Patient Safety: What Healthcare Providers Need To Know
Views: 223 Author: Site Editor Publish Time: 2025-03-28 Origin: Site
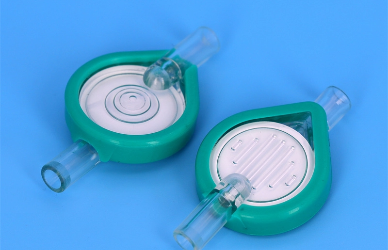
Intravenous (IV) therapy has become a staple in modern healthcare, supporting a broad spectrum of patient needs—from rapid fluid resuscitation in trauma units to carefully titrated drug delivery in oncology wards. Yet, as ubiquitous as IV lines may be, their safety hinges on one critical component that often goes underappreciated: the infusion filter. Not all filters are created equal, and selecting the right model for each clinical scenario is essential to optimize patient outcomes.
Introduction: One Size Does Not Fit All
In a busy hospital environment, IV therapy is deployed across many departments: the intensive care unit (ICU), operating room (OR), oncology suite, pediatric ward, and beyond. Each setting presents unique challenges. For example, a trauma patient in shock may require high-volume fluid administration at rapid rates, whereas a neonate in the neonatal intensive care unit (NICU) demands precise, ultra-low-flow infusions. Meanwhile, oncology patients often receive chemotherapeutic agents that are sensitive to adsorption and precipitation.
Infusion-filter manufacturers offer dozens of models distinguished by membrane pore size, material composition, housing design, and auxiliary features such as air-eliminating chambers. Without careful selection, a filter may either impede flow—jeopardizing hemodynamic stability—or fail to trap harmful particulates and microbes. In the following sections, we'll break down key considerations for different care areas and explain why matching filter specifications to clinical needs is non-negotiable.
Infusion Filters for Critical Care (ICU/CCU)
High Flow Rate Requirements. In critical care settings, patients often present with hypovolemia, hemorrhagic shock, or severe sepsis. Rapid fluid resuscitation—often exceeding 200 mL/hour and sometimes approaching several liters per hour—is required to restore perfusion. Filters used in these scenarios must offer minimal flow resistance. Filters with larger effective surface area, low-resistance polyethersulfone (PES) membranes, and wide-bore housings help ensure that high infusion rates are maintained without undue pressure buildup.
Air Elimination Priority. Critically ill patients, especially those on mechanical ventilation, are prone to fluctuations in vascular pressures. Even small air bubbles can cause an air embolism in compromised cardiopulmonary circuits. Therefore, air-eliminating filters—equipped with hydrophobic vents and air-trapping chambers—are essential. These devices capture and divert microbubbles, allowing clinicians to visually confirm bubble removal before fluid enters the patient’s circulation.
Anticoagulant Compatibility. Many ICU patients receive continuous infusions of heparin or low-molecular-weight heparin (LMWH) to maintain catheter patency or for venous thromboembolism prophylaxis. Standard filters can become clogged when exposed to high concentrations of heparin, potentially reducing flow or causing membrane deterioration. Selecting filters with heparin-compatible housings and membrane materials—such as PES or nylon blends that resist protein binding—helps maintain consistent performance. Additionally, filters labeled “heparin bypass” are specifically tested to ensure no loss of anticoagulant efficacy.
Infusion Filters in Oncology Settings
Chemotherapy Drug Compatibility. Oncology patients often receive medications with complex formulations—liposomal carrier systems, nanoparticle-based drugs, and viscous cytotoxic solutions. A filter's membrane material must be chemically inert to these agents. For instance, polycarbonate (PC) or ethylene-vinyl alcohol (EVOH) membranes may adsorb lipophilic compounds, reducing drug potency. Instead, filters with medical-grade polyethylene (PE) or specially coated PES membranes minimize adsorption and preserve the intended chemotherapeutic dose.
Adsorption and Effective Drug Concentration. Even a small percentage of drug loss to the filter membrane can alter pharmacokinetics, especially with narrow therapeutic-index drugs like vincristine or cisplatin. Filters with validated low-adsorption ratings—often quantified in manufacturer specifications as “≤ 2% drug retention”—ensure that the full drug concentration reaches the patient. Clinicians should consult filter data sheets and choose models tested specifically with chemotherapy agents or specialty infusions.
Long-Duration Infusions. Extended infusions—such as 24-hour continuous infusions of 5-fluorouracil—require filters that resist clogging over time. An oncology filter should have an adequate membrane surface area (often ≥ 10 cm²) to handle high-volume, long-duration flow without rapid pressure spikes. Some filters also incorporate pre-filter layers (e.g., stainless-steel mesh) to trap larger particulates before the primary membrane, extending filter life. Monitoring transmembrane pressure and scheduling proactive filter changes (e.g., every 12–24 hours) can prevent unexpected occlusions.

Pediatric and Neonatal Applications
Ultra-Low Dead Space Design. Neonates and infants have extremely small total blood volumes, making fluid precision critical. A standard adult filter may contain a dead space (the volume between filter inlet and outlet) of 1 mL or more—unacceptably high when a neonate requires only 0.5 mL/hour of fluid. Specialized pediatric infusion filters reduce dead space to as little as 0.2 mL, ensuring that residual fluid in the filter does not lead to dosing errors or unintentional boluses.
Microbubble Detection at Low Flow Rates. At infusion rates below 0.5 mL/hour—common in neonatal parenteral nutrition—air bubbles can remain undetected by standard air-elimination filters. Filters designed for low-flow applications include ultra-sensitive hydrophobic vents and may feature built-in microbubble sensors, which change color upon contact with trapped air. These features help nurses confirm bubble elimination during gravity-drip or syringe-pump infusions, maintaining a bubble-free line critical to fragile preterm infants.
Safety Training and Protocols. Pediatric and neonatal critical care require rigorous protocols. Nurses must be trained to prime filters meticulously—expelling every trace of air—by holding the filter vertically, gently tapping to collect bubbles at the top, and flushing with IV fluid until no bubbles remain. Moreover, filters for these patients are often color-coded (e.g., pink for neonates, yellow for pediatrics) to minimize selection errors. Regular competency assessments, using checklists that cover priming, connection, and occlusion troubleshooting, reinforce best practices and reduce risk.
Nutrition Support (TPN/Lipid Emulsion Infusion)
High-Viscosity Nutrition Challenges. Total parenteral nutrition (TPN) and lipid emulsions pose a dual challenge: high viscosity and particulate content. Lipid emulsions contain microscopic fat droplets (0.2–2 microns) that can coalesce under certain conditions, potentially clogging standard aqueous-solution filters. Filters intended for TPN infusions feature larger pore sizes (1.2 microns or greater) in the outer lipid-resistant layer, coupled with a finer inner layer (0.2–0.5 microns) to trap precipitated proteins or crystalline particles without unduly restricting lipid flow.
Lipid-Affine vs. Hydrophilic Membrane Selection. Lipid-affine membranes—often constructed from polypropylene (PP) or specially treated PES—allow lipid droplets to pass without clinging to the membrane surface. In contrast, hydrophilic membranes repel lipids, causing rapid blockage. When initiating a TPN infusion, filters should be pre-flushed (primed) with a small amount of lipid solution to “condition” the membrane, minimizing the initial binding of fat. Subsequent saline or amino-acid solution priming then readies the filter for a mixed TPN infusion. Collaborative protocols between pharmacy and nursing ensure filters are loaded in the correct sequence, reducing the likelihood of clogging.
Filter Replacement for Long-Term TPN. Patients on long-term TPN—whether at home or in the ICU—require scheduled filter changes to prevent occlusion and maintain infusion accuracy. A typical recommendation is to replace the TPN filter every 24 hours or after 1,000 mL of infusion, whichever comes first. Electronic infusion pumps can alert caregivers to increased pressure differentials, indicating impending filter clogging. Proactive changes minimize pump alarms, prevent unexpected interruptions, and maintain the intended nutrient delivery.
Specialty Infusions (Blood Products, Dialysis Prep, Extracorporeal Circuits)
Blood Product Pre-Filtration. Transfusing blood components—packed red blood cells (PRBCs), platelets, or plasma—carries a risk of microclots, platelet aggregates, or residual leukocytes that can trigger transfusion reactions or microvascular occlusions. Pre-infusion filters with pore sizes around 170–260 microns (microaggregate filters) remove clots larger than red cells, while inline leukocyte-reduction filters (typically 0.5–1.2 microns) eliminate white blood cells and their cytokines. Selecting the correct filter type—microaggregate for trauma resuscitation, leukoreduction for immunocompromised patients—optimizes safety.
Pre-Operative Dialysis and Extracorporeal Circuits. Before initiating hemodialysis or cardiopulmonary bypass, blood often passes through a rapid microaggregate filter to remove microemboli that could damage downstream pumps, oxygenators, or patient vasculature. These high-efficiency filters must withstand flow rates of several hundred milliliters per minute while capturing particles as small as 20–40 microns. Materials such as polyester or nylon netting are favored for their strength and low hemolytic potential.
Drug Compatibility Evaluations. Certain anticoagulants—such as citrate solutions used in dialysis—interact with filter membranes, potentially altering filtration efficiency. Thrombolytic agents (e.g., tPA) used in clot-busting protocols may degrade filter materials over extended dwell times. Prior to use, clinicians should consult compatibility charts provided by filter manufacturers or conduct bench-side tests to confirm that neither the drug efficacy nor the filter integrity is compromised. Filters labeled “dialysis-compatible” or “cytotoxic-compatible” indicate manufacturers have tested these devices under intended conditions.

Summary: Matching Filter to Clinical Need
Given the wide array of infusion scenarios—ICU, oncology, pediatrics, TPN, blood products—it is imperative for healthcare institutions to develop an in-house filter selection list tailored to each department's requirements. Key steps include:
Compile a Departmental Filter Matrix. For critical care, list filters with low resistance and air-elimination features. In oncology, identify models with validated low-adsorption membranes. For neonates, specify ultra-low dead-space, low-flow bubble-detecting filters. Nutrition teams should select lipid-resistant PES/PP designs, while blood-bank staff require microaggregate and leukoreduction filters.
Emphasize Rigorous Product Testing. Ensure filters have documented bacterial-filtration efficiency (e.g., ≥ 99.999% at 0.2 microns), flow-rate specifications tested at clinically relevant pressures, and compatibility certificates for common drugs and solutions. Regularly audit filter performance in clinical practice, tracking occlusion rates, backpressure alarms, and replacement frequency to refine selection criteria.
Develop Combo Procurement Packages. Bundling filters, tubing, and connectors into scenario-specific kits streamlines nursing workflows and reduces selection errors. For example, an “ICU Rapid-Resuscitation Kit” might include a large-surface-area PES filter, standard PVC tubing, and a high-flow connector, while an “Oncology Continuous-Infusion Kit” could pair a low-adsorption PES filter with specialized Luer-lock extensions.
By aligning filter characteristics with patient needs—rather than defaulting to a generic model—healthcare providers can minimize infusion-related complications, reduce nursing burdens, and ultimately improve clinical outcomes. No single filter meets every requirement, but a carefully curated selection list, underpinned by robust product testing and interdisciplinary collaboration, ensures that every patient receives the safest possible infusion therapy.
For more information on selecting infusion filters tailored to your clinical environment, contact ZhenFu Group's technical support team or consult our online compatibility guides.
Related Products
Related News
content is empty!
CONTACT US
NO.176, Gaoxin 5th Road, High-tech Industrial Park, Rizhao City276800, Shandong Province, China +86-13396234532 +86-13396234532Copyright © 2023 ZhenFu Group All Rights Reserved. Technology By leadong.com | Sitemap | Privacy Policy


















